AQA A-Level Physics/Atomic structure
The atom as we know it, was not originally known as it is today. As you may know from GCSE physics, the way in which an atom is structured consists of a nucleus and electrons -- this isn't far from the truth, but there's some differences in the way in which the atom is laid out, and to best understand this, we need to look at how the modern structure of the atom was discovered.
10.1.1 Constituents of the atom -- "What is the atom made of"
The Gold foil test

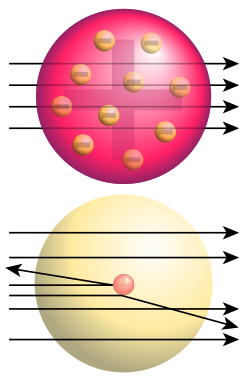
Originally the atom wasn't thought as it is today -- it was thought of as a big ball of solid wobbly matter full of charges in random places, that bent around into all kinds of shapes to make up a solid item, and because of the way in which this theory was tested, everyone believed it must be true beccause if you chop anything up into small pieces, thats what you have!
Well, they were very wrong indeed, and Rutherford knew it. In order to prove that this was the case, rutherford got several kinds of metals and tried to hammer them extremely thin -- unfortunately, all the others wouldn't go very thin before they snapped, so he eventually chose gold.
The reason why he hammered it very thin was because he had an experiment set up in which he fired things called alpha particles (labelled with ) through the foil. These particles consisted of 2 protons and 2 neutrons, and weren't thar very highly charged because they were tiny and that's why he chose them.
Rutherford, working with his assistants Geiger(sound familiar?) and Marsden set up an alpha particle emitter, which fired off alpha particles at the gold foil, and an alpha particle counter called a Geiger counter (sound familiar?) which he placed right next to the alpha particle emitter at first. Rutherford was so sure that all the particals would go straight through he didn't even bother performing it himself!
Now, when he fired the particles at the foil:
- The majority (about 98%) of the particles went straight through the foil! Shock horror! They should have bounced off according to the plum pudding model!
- A very small percentage of particles (1-2%) bounced off, but not in the way they should have in the plum pudding model; they went flying off at very precise angles.
So, from this.. what did rutherford find out? Well, he thought that:
- If the alpha particles went straight through, therefore the atom must contain a majority of empty space.
- Some alpha particles scattered off because they must have been repelled -- this means that there must be something which has more charge and mass because of the way the alpha particle was so quickly scattered away from the nucleus.
The now-known structure
As we know now, the atom contains:
- Nucleons (Protons and Neutrons bundled together)
- Electrons
Now, the things to remember about atoms are that the number of protons in the atom defines what the atom is!, so if there's 1 proton in the nucleus, then it's going to be hydrogen, because it's atomic number (number of atoms) is 1! If you want to know what the element is, then look it up on the periodic table. Now, for some quick facts:
- The number of electrons in an atom is equal to the number of protons, due to the charge of 1 proton pulling in 1 electron (in A/S anyway)
- When eletrons are removed from an atom, it becomes an ion. This is called Ionisation.
- When there are more or less neutrons in the nucleus, then an atom is an Isotope.
These are important concepts, as they're the basis of other theories and models that you'll learn later on in the module. Now, remember these definitions.
- Isotopes are atoms of the same element with different masses due to differing numbers of neutrons in their nucleus.
- Ions are atoms which have electrons removed from their outer shells, and have a charge.
| A | X |
| Z |
| 4 | He |
| 2 |
To understand what these mean, you need to know what the top value and the bottom value means.. the top value,
- A is the number of protons AND neutrons in the nucleus of the element, known as nucleon number
- Z is the number of protons in the nucleus.. so, therefore...
- number of neutrons.
Now, with that said, you will need to be able to calculate the masses and charges of these particles, and you will need to use their specific values unlike in GCSE. Don't worry, you dont need to remember them as you will get them in a data sheet at the front of the exam paper. With that said, it wont hurt to remember them!
| Particle | Charge | Mass |
| Proton | ||
| Neutron | NONE | |
| Electron |
Practice Questions
Don't let the wording phase you, and make sure to read and understand what answer the question wants, and what part is just explaining something. To see the answers, look below.
- An isotope of Plutonium-210 is a radioactive isotope, which emits alpha radiaton. Calculate:
- The number of protons
- The number of electrons
- The number of neutrons
- The isotope undergoes an ionisation process which removes 2 electrons from the atom. Calculate the overall charge of the atom.
Answers
- Number of protons= 94, Number of neutrons=116, number of electrons=94
- (2 x 1.60 x10-19) = 3.2 x10-19